KMU 396
MATERIALS SCIENCE
Midterm Examination
Please give brief answers to the following
questions:
Question 1. (18 pts)
a) What type(s) of bonding would be
expected for each of the following materials and why?
§
brass
(a copper-zinc alloy)
§
rubber
§
barium
sulfide (BaS)
§
solid
xenon
§
bronze
(copper–tin alloy)
§
nylon
§
aluminum
phosphide (AlP)
b) The intermolecular bonding for
HF is hydrogen, whereas for HCl, the intermolecular
bonding is
van der
Waals. Which one would have higher a higher boiling point and why?
Question 2. (10 pts)
Beryllium and magnesium, both in
the 2A column of the periodic table, are lightweight
metals. Which would you expect to have
the higher modulus of elasticity?
Explain, considering binding energy
and atom radii and using appropriate sketches of force versus interatomic
spacing.
Question 3. (12 pts)
Below, atomic radius, crystal
structure, electronegativity, and the most common
valence are tabulated, for several elements; for those that are nonmetals, only
atomic radii are indicated.
Element ΔR% Structure negativity
Cu FCC 2+
C -44
H -64
O -53
Ag +13 FCC 0 1+
Al +12 FCC -0.4 3+
Co -2
Cr -2
Fe -3
Ni -3 FCC -0.1 2+
Pd +8 FCC +0.3 2+
Pt +9 FCC +0.3 2+
Zn +4
Which of these elements would you
expect to form the following with copper:
(a) A substitutional
solid solution having complete solubility?
(b) A substitutional
solid solution of incomplete solubility?
(c) An interstitial solid solution?
Question 4. (20 pts)
Circle the correct word in the
following sentence or fill in the blank:
a)
A material in which atomic bonding is predominantly ionic in nature is less
/ more likely to form a noncrystalline solid upon
solidification than a covalent material because covalent bonds are directional
/ nondirectional whereas ionic bonds are directional
/ nondirectional;
it is less / more difficult for the atoms in a covalent material
to assume positions giving rise to an ordered structure.
b) A crystal structure / system is described by both the geometry of, and
atomic arrangements within, the unit cell, whereas a crystal structure /
system is described only in terms of the unit cell geometry. For example, face-centered cubic and
body-centered cubic are crystal structures / systems that belong to the
cubic crystal system.
c) The
vacancy concentration in a crystal structure increases with ______________.
d) The surface energy of a single crystal depends on crystallographic
orientation / number of grain boundaries because the atomic packing
is different for the various crystallographic planes, and, therefore, the
number of unsatisfied bonds will vary from plane to plane.
(b)
The surface energy will be greater / less for an FCC (100) plane
than for a (111) plane because the (111) plane is more / less densely
packed (i.e., has more / less nearest neighbor atoms in the plane); as a consequence, more
/ less atomic bonds will be satisfied for the (111) plane, giving rise to a
lower surface energy.
Question 5. (20 pts)
The following XRD pattern is
obtained for a sample using monochromatic radiation with wavelength of 0.1542
nm.
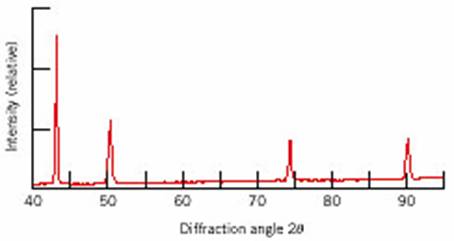
a) Determine the interplanar
spacing for each of the peaks
b) Index each of the peaks (Determine
the indices h, k, l)
c) Determine the crystal structure and
select which of the following materials this sample could be: Fe, Cu, Zn
d) Determine the lattice parameter and
the atomic radius
Question 6. (20 pts)
Assume the following experimental
mechanical testing results are obtained.
Table
1: Rockwell Hardness Test – Scale B
|
|
Test
1 |
Test
2 |
Test
3 |
Test
4 |
Test
5 |
|
Aluminum (Al) |
41 |
40 |
41 |
42 |
41 |
|
Brass (Cu-Zn) |
60 |
65 |
62 |
63 |
62 |
|
Cold Rolled Steel (Fe) |
95 |
98 |
97 |
94 |
98 |
|
Hot Rolled Steel (Fe) |
75 |
74 |
72 |
72 |
73 |
Table 2:
Charpy Impact Toughness test (ft.lbf)
|
Temperature |
T = -200ºC |
T = 25ºC |
T = 200ºC |
||||||
|
Test Number |
Test 1 |
Test 2 |
Test 3 |
Test 1 |
Test 2 |
Test 3 |
Test 1 |
Test 2 |
Test 3 |
|
Aluminum (Al) |
30 |
35 |
33 |
34 |
35 |
33 |
36 |
35 |
35 |
|
Brass (Cu-Zn) |
12 |
11 |
12 |
12 |
12 |
10 |
11 |
11 |
13 |
|
Cold Rolled Steel (Fe) |
1 |
2 |
2 |
45 |
46 |
45 |
59 |
57 |
57 |
|
Hot Rolled Steel (Fe) |
2 |
1 |
1 |
63 |
62 |
64 |
89 |
87 |
90 |
a) How would you list these materials
in terms of hardness starting with the hardest? How would you relate your
answer with the crystal structure of these materials?
b) Which of these materials have a
ductile-to-brittle transition, why do the others do not show such a transition?
How would you relate your answer with the crystal structure of these
materials?
c) Which of these materials would you
select for a knife-blade and why (Explain what type of properties you would
look for in a knife-blade)?
d) Which of these materials would you
select for a regular construction nail and why (Explain what type of properties
you would look for in a nail)?